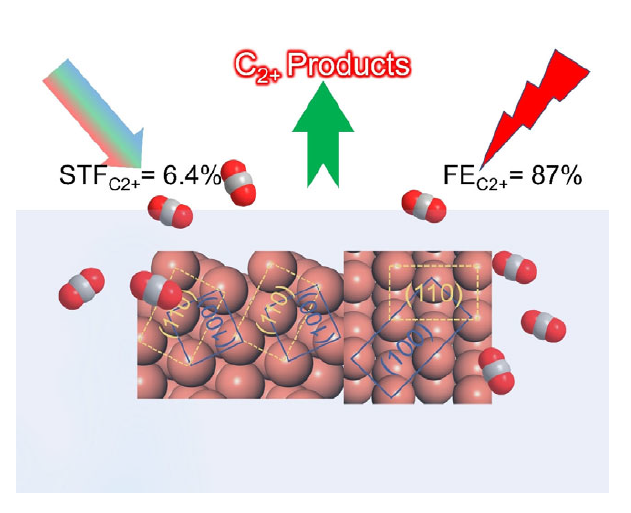
Copper can efficiently electro‐catalyze carbon dioxide reduction to C2+ products (C2H4, C2H5OH, n‐propanol). However, the correlation between the activity and active sites remains ambiguous, impeding further improvements in their performance. In this paper, the facet effect of copper crystals to promote CO adsorption and C−C coupling and consequently yield a superior selectivity for C2+ products is described.
Read more in our recent publication:
Coupling of Cu(100) and (110) Facets Promotes Carbon Dioxide Conversion to Hydrocarbons and Alcohols